Pillbugs & Sowbugs: Isopoda Checklist
Pillbugs, sowbugs, roly-polies and woodlice, collectively referred to as isopods, are land-dwelling crustaceans that feed mainly on decaying organic matter, especially rotting wood and leaves. Pillbugs can roll into a tight ball or a pill — an ability called conglobation. Sowbugs, on the other hand, cannot conglobate and would have a pair of tail-like appendages (uropods) that project out from the rear end of their body.
In conjunction with this post, I have also started a separate Instagram account @isopodsite and a dedicated website for isopods at isopod.site which will have similar content but with more articles specific to isopods. Still lots of content to add and work on, but do check them out if you are interested in terrestrial isopods!
Camera Equipment
I’ve received many enquiries about the equipment that I used for photos on this page so here it is. Cheaper than the latest iPhone, I think.
- Olympus OM-D E-M10 Mk4
- Laowa 50mm f/2.8 2X Ultra Macro APO Lens for Micro Four Thirds
- Godox TT350
- DIY dual-layer diffuser and reflector. Refer to Laowa 50mm 2:1 review
Isopod Biology
As crustaceans, isopods breathe through plate-like gills located on the underside of the abdomen and require very moist environments. They are the only crustaceans living their entire life on land. The females have marsupial pouches on the anterior underside of their body where their eggs and hatchlings are held before being released. Isopods moult in 2 stages: the back half sheds its exoskeleton first, then the front half could take place hours or even days later. The lifespan of most species of isopods is about 2 to 3 years. Most are nocturnal and remain hidden under some form of cover for most of their lives.
Isopod Anatomy
Mature isopods have 7 pairs of walking legs that are pretty much the same. In Latin, “iso” means “same” or “equal” while pod means “foot”. However, isopod mancae (babies) have only 6 pairs of legs in their initial instars. Isopods also have 2 pairs of antennae — one large pair and a tiny pair that is normally not visible. The larger antennae are segmented into 5 peduncles and a flagellum, which may be further segmented into 2 or more articles.
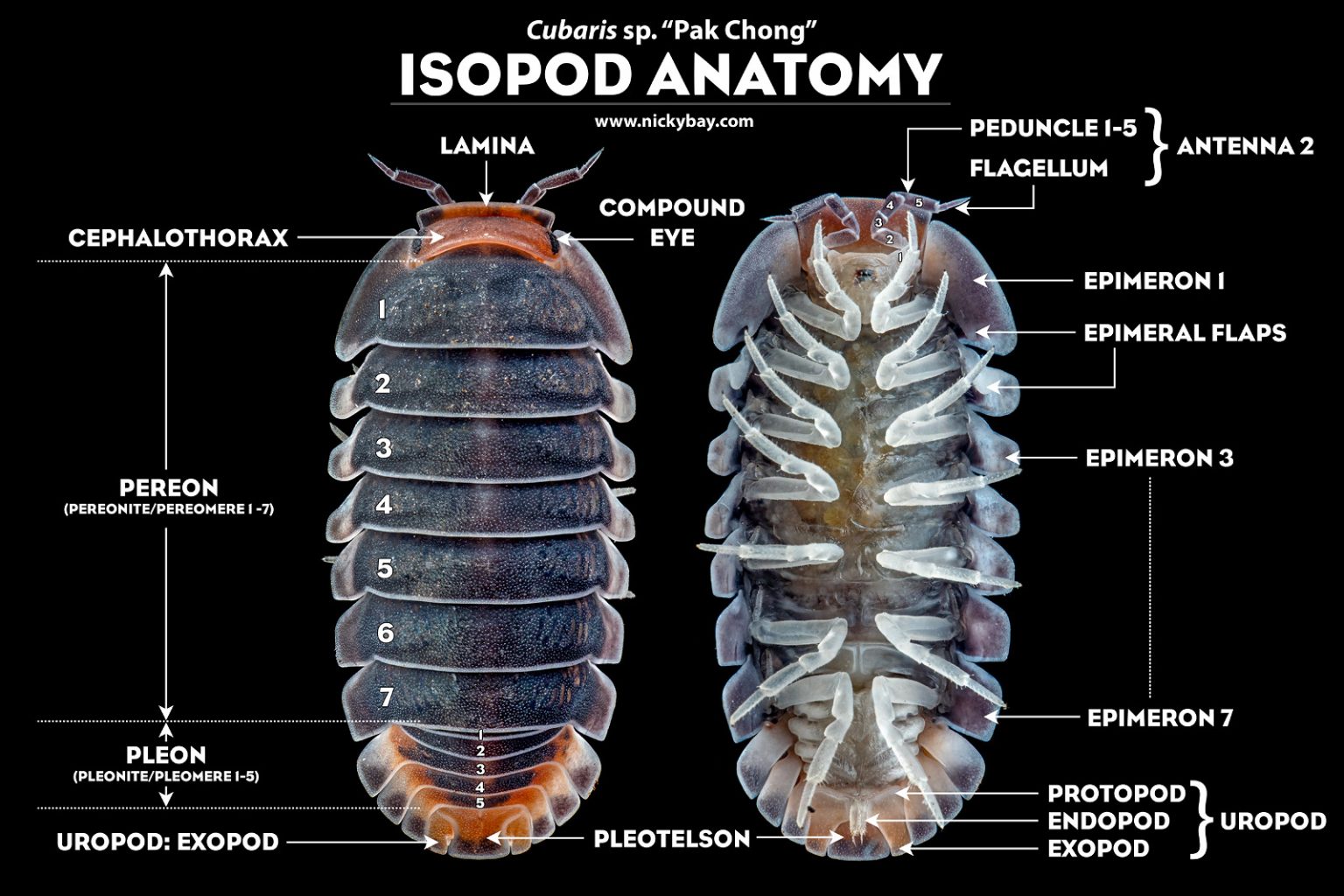
Basic Isopod Anatomy of Cubaris sp. “Pak Chong”
The isopod body consists of a head (cephalon), a thorax (pereon) with 7 segments (pereonites), and an abdomen (pleon) with 6 segments (pleonites) where the last segment is fused with the telson to form the pleotelson. The head is fused with the first segment of the thorax to form the cephalothorax.
Sexing Isopods
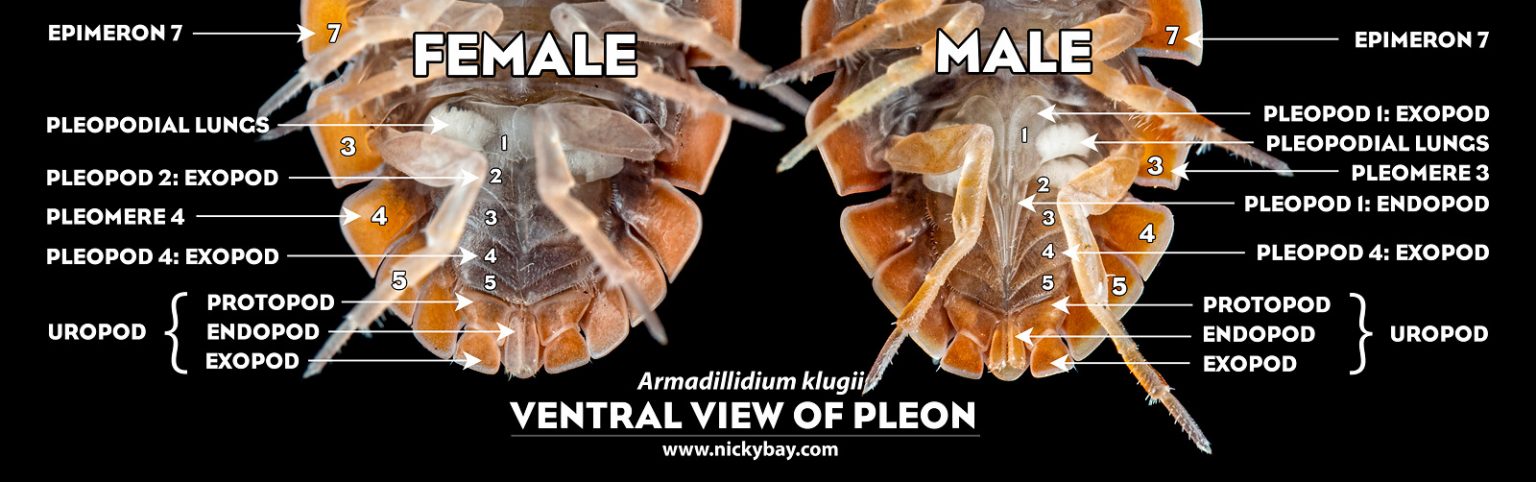
Differences between male and female isopods
Only a few species of isopods are sexually dimorphic. While some may exhibit size differences (the female is often larger), we usually need to look at the pleon’s ventral view to sex them. Males have a pair of long and slender endopods of the first 2 pleopods which are modified to serve as copulatory organs.

Long, slender uropods of a male Porcellio duboscqui troglophila
For some species, the males are easily recognised by their long, slender uropods.
Isopod Reproduction

A female Armadillo tuberculatus being mounted by 3 males simultaneously
The female isopods will release pheromones to signal that they are ready to mate – this may happen after a moult. The sexually mature male isopods will then initiate copulation by mounting the female in a nuptial ride and guarding her against other males while waiting for her to be receptive. This nuptial ride can sometimes take days. He inseminates her gonopores during intermoult or just before a growth moult.

Different stages of development under the marsupium of Armadillidae gen. sp. “Hedgehog”
The eggs are then formed in the brood pouch, which we often refer to as a marsupium. Despite the name, they are most definitely not marsupials. 🙂 The gestation may take a month before the mancae are visible under the brood pouch. When ready, the mancae will finally exit the marsupium for their first moult. During this, they still have only 6 pairs of legs and will only grow their 7th pair in subsequent moults.

Laureola sp. “Ivory Spiky” with freshly released mancae in midst of their initial moult
The mancae may hang around the mother for a day or two if undisturbed. It is not verified if the mother provides any form of maternal care or protection for all species, but it has been reported that Porcellio hoffmannseggi mothers will fiercely protect their young.

Laureola sp. “White Striped Spiky” with mancae hanging around for about a day
Some species of Philosciidae carry their mancae on their back during their initial instars. After the mother moults, the mancae may stick around to feed on her exoskeleton for a calcium boost.

Burmoniscus sp. with mancae clinging on to her back like riding a school bus

Burmoniscus sp. mancae feeding on their mother’s exoskeleton while she completes her moult
Some species of isopods such as Nagurus cristatus and Trichorhina tomentosa are known to be parthenogenetic, which means that only the females are known and they are capable of reproduction without males.

Trichorhina tomentosa releasing a brood of mancae
Moulting and Growth
As described above, mancae start with 6 pairs of legs and form their 7th pair after their initial moults. Moulting, or shedding of its exoskeleton, is necessary for isopods to grow. Like many other invertebrates, their hard exoskeleton cannot expand, so their growth is defined by stages after each moult.

Armadillidae gen. sp. “Choco Drizzle” shedding its posterior half
Isopods moult in two stages where the posterior half moults first, followed by the anterior half. This biphasic moulting allows the non-moulting half to carry out essential functions such as oxygen consumption, preventing water loss, and also to quickly escape predators.

Armadillidae gen. sp. “Choco Drizzle” with its 2 separate halves of shedded exoskeleton
Conglobation
Isopods of several families can roll up into a ball or pill, which we call conglobation. It is an effective defence mechanism against other smaller predators like spiders and ants. It is also the reason why many isopods are referred to as pill bugs or roly polies. On the other hand, species which are unable to conglobate typically have protruding uropods and are commonly called sow bugs.

Armadillo officinalis conglobates readily when disturbed
Acknowledgements
Many photos of isopods from this page were made possible with the kind assistance of isopod enthusiasts, namely Isopods of Singapore, isewpods, live_vine, Brent, ezyeddie, invertebrate dude and many others.
Most identifications were also made with help from Giorgios Agakapis, Philipp Byzov, Nathan Jones, Grant Wang, Matteo Ganz, and many others.
Checklist
This page consists of a personal checklist of all Pillbugs or Sowbugs (Isopoda) that I’ve encountered over the years, as well as those that I’ve documented from isopod breeders.
Localities listed would be the type locality where applicable. Otherwise, it would be the country where the specimens were collected or photographed.
Do note that many genera are poorly defined and difficult to identify. Several species are popularly placed under certain genera (e.g. Cubaris, Merulanella, etc.) in the pet trade but are often incorrect. These will be conservatively placed under a higher taxon (family) when the genus is indeterminate.
This page will be updated regularly, please let me know if you spot any mistakes.
View my isopod Flickr photo album:
References
- World List of Marine, Freshwater and Terrestrial Isopod Crustaceans – Isopoda
- Isopod Zoology: Biology, Husbandry, Species, and Cultivars by Orin McMonigle
- British Myriapod and Isopod Group – Woodlice & Waterlice Checklist
Region Filters
Click on any region to show only records from that region.
Phylum: Arthropoda von Siebold, 1848
Subphylum: Crustacea Brünnich, 1772
Order: Isopoda Latreille, 1817
Suborder: Oniscidea Latreille, 1802
Family: Agnaridae Schmidt, 2003
Genus: Agnara Budde-Lund, 1908

Madagascar: Agnara madagascariensis (Budde-Lund, 1885)
Genus: Hemilepistus Budde-Lund, 1879
- Body long, convex, grey or greyish-brown in colour with lighter tuberosity
- Head and anterior peraeon tergites with armature of conicle tubercles, which may be small and rounded or developed into large prominent crests
- Lateral lobes on head small and set at an oblique angle
- Eyes large, convex
- Median frontal lobe on head small or absent
- Pleon small, narrower than peraeon, smooth dorsally
- Telson triangular with rounded apex
- Antenna 2 short, with strongly developed peduncle and small, two segmented flagellum
- Exopods of pleopods large, expanded
- 2-5 prs of pseudotracheae

China: Hemilepistus sp.
Genus: Orthometopon Verhoeff, 1917
Family: Alloniscidae Schmidt, 2003
Genus: Alloniscus Dana, 1854

Singapore: Alloniscus sp.
Family: Armadillidae Brandt, 1831
Armadillidae diagnosis based on Lillemets & Wilson, 2002
- cephalon compressed longitudinally, with a wide frontal shield
- body able to conglobate
- pleotelson with quadrangular distal part
- antennal flagellum consisting of two articles
- maxillula inner lobe with two robust plumose setae
- male pereonite 7 sternite with bilobed lamellar process
- pseudotracheae on all pleopodal exopods (only on the first four in Buddelundia)
- uropodal protopod flattened with concave medial margin
- uropodal exopod reduced, inserted dorsally near protopod medial margin
Genus: Armadillo Latreille, 1802
Armadillo diagnosis translated from MATTERN, D. (1999) Die Gattung Armadillo im westlichen Mittelmeergebiet (Isopoda Oniscidea Armadillidae)
- Eusphere, endo-antennae Kugler
- Pleopod exopodites 1-5 with lungs
- Epimers of the 1st pereionite with schism and lateral groove
- Epimers of the 2nd pereionite split into an inner and an outer lobe
- Propodus of the pereiopods IV and V in both sexes with a plectrum (= striated ridge)
- Telson hourglass-shaped, with an incision at the level of the uropod exopodite
Genus: Calmanesia Collinge, 1922

Madagascar: Calmanesia erinaceus Barnard, 1958
Genus: Cubaris Brandt, 1833
Cubaris is considered a non-monophyletic group. Many species in this group are only provisionally placed under Cubaris and should belong to other genera if a comprehensive revision of the genus is done.
Cubaris diagnosis based on Lillemets & Wilson, 2002
- frontal lamina not raised above vertex, midline not indented
- antennae slender
- dorsal surface smooth, rugose or tuberculate, but without spines
- epimera tergite junctions 1–6 posterior margins more or less incurved, tergite 7 junction straight or shallowly incurved
- epimera 1 posterior margin entire, not cleft
- epimera 1 endolobe small, not visible dorsally, not forming continuation of epimera margin
- epimera 2 endolobe not projecting beyond epimera margin
- tergite 1 length 0.2–0.25 pereon length
- pleotelson sides parallel or constricted, dorsal surface not keeled, posterior margin bluntly rounded, straight or shallowly incurved, not deeply incised in midline
- pleopods width greater than 0.3 pleon width
- proximal portion length less than 0.3 protopod length, inner margin near exopod insertion smoothly concave

Brazil: Cubaris murina Brandt, 1833

Thailand: Cubaris sp. “Pak Chong”

Thailand: Cubaris sp. “Panda King”

Martinique: Cubaris sp. “Salmon Orange”
Genus: “Cubaris” (indet)

Thailand: “Cubaris” sp. “Amber Ducky”

Thailand: “Cubaris” sp. “Amber Panda”

Thailand: “Cubaris” sp. “Apricot”

Thailand: “Cubaris” sp. “Blue Pigeon”

Thailand: “Cubaris” sp. “Bumblebee”

Thailand: “Cubaris” sp. “Cappuccino”

Thailand: “Cubaris” sp. “Caramel Creme”

Thailand: “Cubaris” sp. “Copper”

Thailand: “Cubaris” sp. “Emperor Bee”

Thailand: “Cubaris” sp. “Firefly”

Thailand: “Cubaris” sp. “Green Laser”

Thailand: “Cubaris” sp. “Happy Nun”

Thailand: “Cubaris” sp. “Hong Tiger”

Thailand: “Cubaris” sp. “Jupiter”

Thailand: “Cubaris” sp. “Lemon Blue”

Thailand: “Cubaris” sp. “Mango Sticky Rice”

Thailand: “Cubaris” sp. “Opal”

Thailand: “Cubaris” sp. “Purple Potato”

Japan: “Cubaris” sp. “Red Edge”

Thailand: “Cubaris” sp. “Rubber Ducky”

Thailand: “Cubaris” sp. “Blonde Ducky”

Thailand: “Cubaris” sp. “Sun Tiger”

Thailand: “Cubaris” sp. “White Ducky”

Thailand: “Cubaris” sp. “White Shark”

Thailand: “Cubaris” sp. “Yellow Tiger”

Malaysia: “Cubaris” sp. “Giant Raichu”

Thailand: “Cubaris” sp. “Flame White Ducky”
Genus: Dryadillo Taiti, Ferrara & Kwon, 1992
Dryadillo diagnosis based on Taiti, Ferrara & Kwon, 1992.
- Dryadillo is characterised by the cephalon with the frontal lamina not protruding above vertex;
- epimeron of pereonite 1 with lateral margin not thickened, posterolateral corner with a shallow schisma with inner lobe much shorter than outer one (see Figs 27d, 28d);
- pereonite 2 with semicircular ventral lobe (see Figs 27d, 28d and fig. 86 in Herold 1931);
- telson with distal part narrower than basal;
- uropodal protopod with dorsal medial tooth and short exopod.

Unknown: Dryadillo sp. “Dream”
Genus: Laureola Barnard, 1960

Vietnam: Laureola sp. “Bumble Bee Spiky”

Vietnam: Laureola sp. “Ivory Spiky”

Vietnam: Laureola sp. “Panda Spiky”

Vietnam: Laureola sp. “White Striped Spiky”

Vietnam: Laureola sp. “White Skull Spiky”
Genus: Merulanella Verhoeff, 1926
Almost all species listed in this genus do not belong to Merulanella, but are commonly presented as Merulanella. The actual genera have yet to be determined.

Vietnam: “Merulanella” sp. “Milky Edge”

Vietnam: “Merulanella” sp. “Ember Bee”

Vietnam: “Merulanella” sp. “Tricolor”

Vietnam: “Merulanella” sp. “Blister”

Vietnam: “Merulanella” sp. “Red Diablo”
Genus: Nesodillo Verhoeff, 1926
Nesodillo is characterized by the semicircular ventral lobes on pereonal epimera 1-3.
This group contains species which may be in Nesodillo but are also candidates for other similar genera like like Triadillo.

Taiwan: Nesodillo sp. “Shiro Utsuri”

Malaysia: Nesodillo sp. “White Tiger”
Genus: Parakermania Vandel, 1973
Genus: Reductoniscus Kesselyak, 1930
Reductoniscus diagnosis based on Kesselyak, 1930.
- Right mandible with a chewing tooth, inside a large and two small penicillia (Fig. 17).
- Left mandible with two chewing teeth, one large and three small penicillia (Fig. 18).
- Inner branch of the anterior maxilla highlighted by two brushes. I. and II.
- Epimers split.
- The groove created by the duplication of the epimer continues under the front strip.
- The pleon epimers and the telson enclose themselves in them, since the animals are perfect spheres.
- Head and pereion tergites with cusps and ribs stronger on the posterior segments. I. and II.
- Pleontergites are absent, accordingly the sternites are also reduced.
- In (3 ‘and 9 there are only four pleopod exopodites. In j, however, the I. pleopod endopodites are formed.
- The last two pleopod exopodites have a small, silvery tracheal field at g and outside.
- Secondary gender characters are unavailable.

Singapore: Reductoniscus costulatus Kesselyak, 1930
Genus: Sinodillo Kwon & Taiti, 1993

Vietnam: Sinodillo sp. “Vietnam”
Genus: Spherillo Dana, 1853
Genus: Troglodillo Jackson, 1937
Troglodillo diagnosis based on Jackson, 1937.
- Head of Armadillo type ; well developed frontal shield, anteroposteriorally flattened.
- Maxillula : inner endite with two very short and stout penieilli no terminal spine; outer endite 4 + 6 (all simple and long).
- Maxillipede : endopod very short ; endite with three large terminal spines.
- Margin of first thoracic tergite neither split nor grooved; articulating laciniae absent on all tergites.
- First and second abdominal tergites very short and without postern-lateral angles ; remainder well developed.
- Base of uropod drawn back ; exopod small. Telson broad, exceeding uropod.
- First pleopod of female without opercular portion, only tracheal part being present.

Thailand: Troglodillo sp. “Lan Saka Tiger”

Thailand: Troglodillo sp. “Soil”

Malaysia: Troglodillo sp. “Purple Giant”

Vietnam: Troglodillo sp. “Cave”

Malaysia: Troglodillo sp. “Yellow Dust”
Genus: Venezillo Verhoeff, 1928

Singapore: Venezillo parvus (Budde-Lund, 1885)
Unidentified Armadillidae

Thailand: Armadillidae “Thunder Dragon”

Australia: Armadillidae “Hieroglyph”

Guadeloupe: Armadillidae “Guadeloupe”

Singapore: Armadillidae “Sentosa”

Singapore: Armadillidae “Mini Purple”

Singapore: Armadillidae “Platin Tung Song”

Thailand: Armadillidae “Draco Spiky”

Thailand: Armadillidae “Hedgehog”

Thailand: Armadillidae “Fancy Gator”

Thailand: Armadillidae “Shiny Gator”

Singapore: Armadillidae “Singapore Tiger”

Vietnam: Armadillidae “Wasp”

East Malaysia: Armadillidae “Mulu Mini”

East Malaysia: Armadillidae “Cave Tiger”

East Malaysia: Armadillidae “Cave Tiger Orange”

East Malaysia: Armadillidae “Cave Tiger Vanilla”

Thailand: Armadillidae “Big Blue”

Thailand: Armadillidae “Platinum Ducky”

Guadeloupe: Armadillidae “Choco Drizzle”

Thailand: Armadillidae “Devil’s Mask”
Family: Armadillidiidae Brandt, 1833
Genus: Armadillidium Brandt, 1833
Armadillidium is recognised by their triangular telson and truncated “square” uropods that end flush with the body and their abilty to roll into a ball.

United Kingdom: Armadillidium album Dollfus, 1887

United Kingdom: Armadillidium depressum Brandt, 1833

Montenegro: Armadillidium klugii Brandt, 1833 “Clown”

Croatia: Armadillidium odhneri Verhoeff, 1930

Germany: Armadillidium opacum (C. Koch, 1841)

Croatia: Armadillidium pallasii Brandt, 1833
Synonymised from Armadillidium frontirostre Budde-Lund, 1885

Montenegro: Armadillidium pallasii Brandt, 1833 “Kotor”
Synonymised from Armadillidium frontirostre Budde-Lund, 1885

Greece: Armadillidium peraccae Tua, 1900

Montenegro: Armadillidium scaberrimum Stein, 1859 “Kotor”

Slovenia: Armadillidium versicolor Stein, 1859

United Kingdom: Armadillidium vulgare Latreille, 1804 “Magic Potion”

United Kingdom: Armadillidium vulgare Latreille, 1804 “St Lucia”

United Kingdom: Armadillidium vulgare Latreille, 1804 “Big Spot”
Genus: Cristarmadillidium Arcangeli, 1936
Genus: Echinarmadillidium Verhoeff, 1901
Genus: Eluma Budde-Lund, 1885

French Guiana: Eluma caelata (Miers, 1878)
Family: Cylisticidae Verhoeff, 1949
Genus: Cylisticus Schnitzler, 1853

Netherlands: Cylisticus convexus (De Geer, 1778)
Family: Delatorreiidae Verhoeff, 1938
Genus: Pseudarmadillo Saussure, 1857
Family: Eubelidae Budde-Lund, 1899
Genus: Elumoides Taiti & Ferrara, 1983

Singapore: Elumoides sp. “Soil White”

Thailand: Elumoides sp. “Soil Yellow”

Singapore: Elumoides sp. “Soil Orange”
Genus: Mesarmadillo Dollfus, 1892

Nigeria: Mesarmadillo sp.
Family: Ligiidae Leach, 1814
Genus: Ligia Fabricius, 1798

Singapore: Ligia sp.
Family: Oniscidae Latreille, 1802
Genus: Oniscus Linnaeus, 1758

Netherlands: Oniscus asellus Linnaeus, 1758
Family: Philosciidae Kinahan, 1857
Genus: Anchiphiloscia Stebbing, 1908
Genus: Burmoniscus Collinge, 1914
Genus: Chaetophiloscia Verhoeff, 1908

Montenegro: Chaetophiloscia elongata (Dollfus, 1884) “Kotor”
Genus: Philoscia Latreille, 1804

Netherlands: Philoscia muscorum (Scopoli, 1763)
Unidentified Philosciidae

East Malaysia: Philosciidae “Lagang Cave”

East Malaysia: Philosciidae “Lemon Blue Mini”
Family: Platyarthridae Verhoeff, 1949
Genus: Cephaloniscus Ferrara & Taiti, 1989
Genus: Niambia Budde-Lund, 1904

South Africa: Niambia capensis (Dollfus, 1895)
Genus: Trichorhina Budde-Lund, 1908

Singapore: Trichorhina sp.
Family: Porcellionidae Brandt & Ratzeburg, 1831
Genus: Agabiformius Verhoeff, 1908
Genus: Leptotrichus Budde-Lund, 1885

Guadeloupe: Leptotrichus cf. panzerii (Audouin, 1826)
Genus: Porcellio Latreille, 1804

Europe: Porcellio laevis Latreille, 1804

Croatia: Porcellio obsoletus “Split”

Europe: Porcellio scaber Latreille, 1804

Greece: Porcellio spinicornis Say, 1818

Canary Islands: Porcellio sp. “Spiky Canare”

Canary Islands: Porcellio sp. “Spiky Canare Ivory”
Genus: Porcellionides Miers, 1878
Family: Scleropactidae Verhoeff, 1938
Genus: Adinda Budde-Lund, 1904

Singapore: Adinda sp. “Pointy Beak”

East Malaysia: Adinda sp. “Gold Striped Gator”
Genus: Circoniscus Pearse, 1917
Genus: Protoradjia Arcangeli, 1955
Protoradjia diagnosis based on FERRARA, F., MELI, C. & TAITI, S. (1995) Taxonomic revision of the subfamily Toradjiinae (Crustacea, Oniscidea Scleropactidae)
- Animals with exoantennate ability to roll up into a ball.
- Cephalon with frontal shield slightly bulbous in the middle and distinctly protruding above vertex, separated from this by a deep groove.
- Pereonite 1 with a very shallow notch (schisma) at posterior corner, that separates two lobes, of which the outer one protrudes distinctly backwards in relation to the inner one.
- Pereonite 2 with transverse ridge on ventral surface of epimera.
- Telson semicircular, distinctly shorter than tips of pleonite 5 and uropodal protopods.
- Antennule of three articles.
- Antennal flagellum of two articles with a long apical organ.
- Mandible with molar penicil simple or semidichotomized;
- right mandible with 1 + 1 penicils and left one with 2 + 1 penicils.
- Maxillule outer branch with 4+ 6 (5 cleft) teeth, a small accessory tooth and a stalk, inner branch with two long penicils.
- Maxilliped with setose endite bearing a penicil. Pereopods with long and plumose bifid or trifid dactylar seta.
- Pereopod 7 basis with a deep groove bordered with scales on rostral surface.
- Pleopods 1-2 exopod with open lungs similar to those in Trachelipus.
- Uropodal protopod flattened;
- exopod inserted in a notch of the medial margin; endopod distinctly surpassing posterior margin of telson.
Unidentified Scleropactidae

Singapore: Scleropactidae “Big Bear”
Family: Trachelipodidae Strouhal, 1953
Genus: Nagurus Holthuis, 1949

Singapore: Nagurus nanus (Budde-Lund, 1908)

Singapore: “>Nagurus cf. cristatus (Dollfus, 1889)
Genus: Trachelipus Budde-Lund, 1908

Germany: Trachelipus rathkii (Brandt, 1833)

Croatia: Trachelipus sp. “Kotor+Dubrovnik”

Croatia: Trachelipus sp. “Hrvatska”
Family: Tylidae Dana, 1852
Genus: Helleria Ebner, 1868
Family: incertae sedis
Genus: Exalloniscus Stebbing, 1911
Exalloniscus diagnosis based on TAITI, S. & CARDOSO, G. M. (2020) New species and records of Exalloniscus Stebbing, 1911 from southern Asia (Malacostraca, Isopoda, Oniscidea)
- Length between 2.7 and 7.5 mm.
- Animals with flattened or moderately convex body;
- body outline without interruption between pereon and pleon.
- Tergites with scattered scalesetae;
- one line of noduli laterales per side, very small and inserted on posterior margins and far from lateral margins of pereonites;
- gland pores absent.
- Cephalon semicircular, flattened in front, with lateral lobes often protruding laterally;
- frontal and suprantennal lines present.
- Eyes reduced or absent.
- Epimera of pleonites 3-5 well developed, directed backwards.
- Telson short, triangular. Antennal flagellum three-jointed.
- Outer branch of maxillula with 11-12 teeth and one or two accessory setae.
- Maxilliped endite with penicil.
- Pereopods with flagelliform dactylar seta, often plumose apically.
- Basis of pereopod 7 usually with water conducting system in form of longitudinal groove on rostral surface covered with lamellar scales (not present in E. maschwitzi and E. vietnamensis).
- Pleopodal exopods without respiratory structures.
- Uropodal endopod inserted proximally to exopod; exopod slightly grooved on outer margin.



































































































Randy Thill
What an education! As far as I have been able to accertain, I’ve seen one kind…plain grey rolly polly that loves to be under rocks and pots in the garden. They always make me smile. Don’t know what I’d do if I saw a “pretty” one or one with spikes. I probably need not worry about that, tho.
Thanks so much for broadening my knowledge. If I ever get to another country, I’ll check under their flowerpots and rocks to see if I can see another kind for real!
Tom van der Ende
Dear NickyBay,
I send you this message because I saw a beautiful picture on the internet what is not on your website itself of a Vietnam: “Merulanella” sp. “Red Diablo” and my question is I have a deadly disssease and medical people don’t know how long I have left I breeding this species also myself but get them never so nice on the picture and I love to use this picture for on my bussines card. I’m NON PROFIT so please can you tell me what are the cost to use a picture from you and is it possible ???? I put a link to the picture I like to use on the place you ask website.
Please let me know if I can use these picture what it will cost I’m non profit